New Scientist,
Why sugar is bad for you
Anna Furth (Lecturer in Biology, Open University), and
John Harding
(Laboratory of Ophthalmology, Oxford University)
Put proteins in a concentrated solution of sugar and you
can watch the transformation. The sugar slowly binds to the proteins,
permanently altering their molecular structure and, as a result, the way they
work. The original incentive to look into this reaction, which is known as
glycation, came from the food industry. There, glycation is bad news, because
proteins that are modified by sugars tend to turn yellowy-brown on standing.
This makes them less nutritious and puts off prospective buyers. It now seems
that we too go yellowy-brown on standing—on ageing, that is. It happens as
excess sugar in the diet slowly attacks proteins in our bodies. We may find
drugs that slow the process, but the best strategy is probably for everyone,
even those without diabetes, to avoid sugary snacks on an empty stomach.
The clearest examples of glycation are in the proteins
of the lens in the eye. If you leave a lens from a human eye in a concentrated
solution of glucose, it goes cloudy and looks like a lens afflicted with
cataract. Diabetes may in effect replicate this experiment in the body, because
the disease raises levels of glucose in the blood. People with diabetes are at
least five times as prone to cataracts as other people. They are also more
likely to suffer from atherosclerosis (the clogging of the arteries with fatty
plaques) and may have problems with their kidneys and their circulation. In
these and other complications of diabetes, the glycation of proteins probably
plays a major role.
Glycated proteins differ sharply from normal, and harmless, glycoproteins. Both carry sugar molecules attached by
covalent bonds, but glycoproteins acquire theirs only
through reactions that are carefully controlled by enzymes. Glycation, on the
other hand, happens spontaneously, at a rate which depends largely on the
concentration of the attacking sugar. Any sugar will do, provided it has a free
carbonyl (C-O) group, but glucose is by far the commonest. Only two types of
chemical group in the amino acids of a protein are vulnerable to glycation: the
free amino group (HN2) on the amino acid lysine, and to the so-called amino
terminal at one end of the protein chain.
As long ago as 1912, a Frenchman called Louis Maillard worked out the three-step reaction that turns
proteins brown when sugars attack them spontaneously. Step 1 produces a compound
known as a Schiffs base; this reaction is easily
reversed by lowering the concentration of glucose. But some Schiffs
base is inevitably converted to Amadori product, a
highly undesirable compound with a reactive carbonyl group. This enables the Amadori product to react with amino groups on other
proteins, cross-linking them irreversibly into large clumps composed of many
molecules. These aggregates are known as Maillard
products by food chemists and AGEs by diabetologists.
(AGE somewhat whimsically stands for advanced glycation end products, as these
are thought to accumulate as we grow older.) The enzymes that usually digest
proteins cannot easily remove AGEs. Some may be
attacked by the body's scavenger cells, macrophages, but this can stimulate unwanted
side effects in the surrounding tissue.
The complications of both diabetes and ageing develop
slowly, and so do glycation and cross-linking. It takes hours for the first
product to build up, weeks for the AGEs. Because this
is an un-catalyzed reaction, its rate is determined largely by two factors: the
concentration of reactants and the length of time the molecules are exposed to
a particular concentration. Both factors are sharply raised in uncontrolled
diabetes, when a meal that is high in carbohydrates floods the blood with sugar
from the gut. Without insulin to speed up the usual mechanisms for distributing
glucose around the tissues, levels of glucose in the blood shoot up and may
stay high for several hours—creating prime conditions for the glycation of body
proteins.
But people with diabetes are not the only ones to be
prone to high levels of glucose. Anyone who eats 50 grams of pure glucose
(about the amount in one-and-a-half Mars bars) on an empty stomach will find
that the levels of glucose in their blood shoot up. This response is portrayed
graphically in a "glucose tolerance curve" that charts the rise over
time (see Figure). The area under the curve,
represents the danger zone for glycation. This zone increases as middle age
creeps on. The older we get, the more pronounced and prolonged the rise in our
blood glucose when we are "challenged" with an influx of glucose.
So glycation is a potential problem for many of us,
not just those who know that they have diabetes. Yet, until recently, medical
researchers remained fairly complacent about its dangers. It was commonly
supposed that most proteins are regularly replaced by fresh, unglycated molecules, in the natural process of
"turnover", before they progress beyond stage 2—the Amadori product. But more recent work shows two reasons why
we should take glycation seriously.

Sweet peril for proteins: the glycation of protein
happens in stages. First, a protein with a free amino group gains a glucose with a free carbonyl. The first reaction is
readily reversible. The second is not. The third reaction is particularly
important in long-lived proteins. It produces cross-links known as AGEs—advanced glycosylation end
products—turning proteins into useless clumps
First, many
proteins do malfunction when they are converted to Amadori
products. For example, glycated albumin, an important
protein in the blood, loses much of its capacity to bind to long-chain fatty
acids. And glycated lipoproteins— which carry
cholesterol in the blood—are no longer recognised by
receptors on the surface of cells. Both these malfunctions could impair the way
the body deals with fat and cholesterol, and so promote the development of
coronary heart disease. A third effect of glycation is that the body's most
abundant antibody, called immunoglobulin G, becomes less able to cope with
bacterial toxins such as streptolysin.
Another reason to worry about glycation is that some
proteins are extremely long-lived. In this case, the turnover of proteins is so
slow that it will not remove Amadori products before
they can be converted to AGEs. Two important types of
long-lived proteins are crystallins in the lens of
the eye and myelin in the fatty insulatory sheath
around nerves. The glycation of myelin could contribute to the nerve damage
that is associated with diabetes: the glvcation of crvstallin,
makes the lens opaque. As with any protein, this is because glycation upsets
the balance of charged groups on the protein's surface, altering the way that
it interacts with water and other molecules. Glycated
molecules of crystallin clump together, excluding
water to give an opaque suspension that is not much good for seeing through.
Researchers into diabetes are also particularly
interested in a third long-lived protein: this is collagen, the structural
protein in skin, tendon, and most importantly, basement membrane. This last is
the critical, selectively permeable material that lines the capillaries, the
filtration units of the kidney and the larger blood vessels. These structures
are often damaged in people suffering the secondary complications of diabetes,
and often also in older people. The collagen making up the basement membrane
has an unusual structure, forming an open, three-dimensional network that holds
the other components of the membrane together. Glycation, at least in the
laboratory, impairs collagen's ability to form this three-dimensional network.
So the way that glucose affects this protein could turn out to be the single
most important, and most unfortunate, of all forms of glycation.
Clearly, then, glycation is best prevented, but how?
An anti-glycation drug is one possibility. We might be able to find a drug that
protects the vulnerable amino groups of proteins in step 1, or one that blocks
the reactive carbonyl group that leads to cross-linking in step 3. Researchers
can indeed prevent step 1, at least in the laboratory, by using aspirin. The
aspirin molecule transfers its acetyl group (CH3CO) to proteins and sometimes
this protects them against glycation. Why it does so is not clear. It is not
always simply a matter of the acetyl group itself binding to the site that
would otherwise be attacked by sugars; aspirin even protects some proteins that
are usually glycated at another site. Whatever the
protection mechanism, the protein altered by aspirin cannot form crosslinks as the Amadori product
does, and this is a big advantage.
The worry is that aspirin itself might cause
structural changes and set off damaging processes. In fact this does not
happen, at least not to lens proteins in the laboratory. Aspirin does not
unfold the protein—one such damaging process—nor cause
the lens to become opaque. On the contrary, aspirin prevents cyanate and some sugars from causing such opacity. Researchers
first showed that aspirin could prevent glycation in people when they studied
albumin in the blood. Later they found that the drug could also protect
proteins in membranes in the retina and in the lens. Three proteins in the eve
are useful test cases in the search for antiglycation
drugs, because their turnover is exceedingly low. Damage accumulates over the
years, eventually developing into cataracts. Although "chemical
insult" by any reactive molecule contributes, the damage that sugar
induces is by far the most common cause of cataract. Recently Ros Ajiboye and Kerry Robens at the Nuffield Laboratory of Ophthalmology in
Oxford have shown that another drug, ibuprofen (prescribed as Brufen and sold as Nurofen), can
decrease glycation in isolated lens proteins. Both aspirin and ibuprofen are
drugs that reduce inflammation.
Insights from lateral thinking
Ibuprofen does not have an acetyl group and therefore
cannot protect by acetylation. So why did researchers
even try it? Edward Cotiier at Cornell University in
New York was the first to claim that aspirin protected people with rheumatoid
arthritis and diabetes against cataract. At the Nuffield Laboratory in Oxford,
we then compared patients with cataracts with people who are free of cataracts
but of a similar age. Ruth van Heyningen at the
Nuffield asked people from both groups what drugs they had taken regularly for
at least four months at any time in the past. The team had designed the study
to look for risk factors—that is, factors which are more common in people with
cataracts than in the controls. Clearly, something that protects against
cataracts would be more common in the people who were free of cataracts than in
the patients. We found that aspirin, paracetamol and
ibuprofen all protect against cataract.
Otto Hockwin and his
colleagues at the University of Bonn, in West Germany, have since confirmed
these findings. A second unpublished study in Oxford showed that even low doses
of these drugs—say one tablet a day for 18 months— reduces the risk of
developing cataracts. The protective effect extended to people with diabetes.
Very recently, H. Mohan and his colleagues in India confirmed the finding that
taking aspirin protects against cataracts.
A common mechanism for all these drugs is difficult to
discern, but it may involve preventing the glycation of the lens protein.
Aspirin and ibuprofen also stimulate the body to produce insulin, which lowers
the level of glucose in the blood.
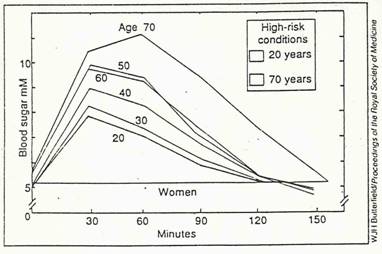
Consume 50 grams of glucose and the levels of glucose
in the blood shoot up. As we age,
dangerous levels persist for longer.
It is worth investigating how those changes come
about, as these drugs could protect other proteins besides those in the lens.
Unfortunately, it tends to be easier to attract money for research into new
drugs than for re-evaluating old ones.
Drugs that interfere at a later stage—the conversion
of Amadori product to AGE—might block the carbonyl
group in the Amadori product. This would prevent the Amadori product from cross-linking to other proteins via
their amino groups. Two drugs that are possible candidates are penicillamine, an antirheumatic
drug, and aminoguanidine. Tony Cerami
and his colleagues at Rockefeller University in New York fed aminoguanidine to diabetic rats for 16 weeks, and found
that this prevented the cross-linking of collagen in the aorta, the main blood
vessel of the heart. Over five months, it also prevented the thickening of
basement membranes which is so characteristic of diabetic complications. People
reportedly show no ill effects after two weeks on aminoguanidine
but, so far, this potential anti-glycation drug has gone no further than an
application for a patent to use the compound "to prevent ageing in food
and animal proteins". Meanwhile, researchers disagree as to exactly where
in the glycation sequence the drug acts.

Simon Wolff at University College, London, takes a
totally different approach to anti-glycation therapy. He feels that glucose
does not damage proteins along the route we have described. Rather, he suggests
that proteins fragment, largely under the influence of toxic free radicals—very
reactive forms of oxygen. These compounds form as glucose spontaneously
combines with oxygen, a process known as auto-oxidation. According to Wolff’s
theory, certain antioxidants such as vitamin C and E, which block this
reaction, could be therapeutic. But such a conclusion is still premature and
controversial. In the laboratory at least, vitamin C itself is an effective glycating agent, proceeding right through to AGEs.
Help from oxygen?
Other research, by John Baynes
and his colleagues at
The idea of an anti-glycation drug is attractive: it
could be an elixir of youth, a cure for diabetic complications, and a goldmine
for the pharmaceuticals industry. Nonetheless, the unpalatable truth is that we
already have a much simpler way of reducing glycation—by eating less sugar.
The consumption of sugar has risen enormously over the
past 200 years. Many diseases, such as coronary heart disease and diabetes,
have become more common over the same period. Although the link between sugar
and disease is hotly contested, there is no disputing the data in the glucose
tolerance curves. Our bodies cannot cope with large influxes of pure sugar. The
level of glucose in the blood rises sharply, and remains high for more than an
hour, particularly as we grow older. The drink containing 50 grams of glucose
that researchers gave people during a glucose tolerance test may not feature on
the menu as such, but there is the same amount of sugar in a half a litre of unsweetened apple or orange juice, and nearly as
much as in a Mars bar. A can of Lucozade contains 65
grams of pure glucose.
The argument against high-sugar
snacking follows directly from the glycation mechanism. Only step 1 of the sequence needs free glucose. A
small proportion of the first product, Schiffs base,
will inevitably turn into Amadori product even in the
absence of glucose. Because this second step is virtually
irreversible. Amadori product will remain in
circulation until the natural turnover of proteins gets rid of it. For
long-lived proteins, such as the crucial collagen of the basement, membranes, Amadori products may remain until AGE crosslinks
form in the final stage of the reaction sequence.

Has the
enormous rise in our consumption of sugar led to disease?
The important point is that you do not need free
glucose, either to turn Schiffs base to Amadori product, or to turn Amadori
product to AGE. This is obvious when you put proteins in a test tube with t;lucose. If you
remove the glucose part way through the incubation. Amadori
product continues to form and cross-linking actually speeds up. Our preliminary
studies at the Open University suggest that just the same thing happens in
people. So brief overexposure to glucose, such as a
high-sugar snack on an empty stomach, could give enough Schiffs
base for small amounts of Amadori product to continue
forming throughout the next few days. If long-lived proteins are
involved, AGEs will eventually result. So by
extrapolating information from the laboratory to people, we can see how
ill-advised snacking could slowly build up proteins damaged by glucose.
If this extrapolation is valid, we can prevent, or at
least reduce, glycation by avoiding the eating habits that produce the glucose
tolerance curves. Those in the high-risk category—that is, elderly people and
those approaching middle-age—might especially profit from the dietary advice
given to people with diabetes: take carbohydrate as part of a mixed meal with
protein, fat and fibre and avoid high-sugar snacks on
an empty stomach.
It is not just table sugar or sucrose that we should
worry about. Sucrose is a disaccharide, split apart during digestion into monosaccharides, glucose and fructose. Both have the free
carbonyl group needed for glycation. Recently, Gerardo Suarez at New York
Medical College has focused on fructose as a possible glycating
agent in people. In the test tube, it can damage proteins more rapidly than
glucose. Haemoglobin— the protein that carries oxygen
in the blood—is glycated five times as rapidly with
fructose as with glucose, and albumin is cross-linked 10 times as rapidly.
Although fructose in food seldom leads to high levels of fructose in the blood,
some of our cells can produce fructose from glucose. As a result, people with
diabetes tend to have high levels of fructose in certain tissues, such as the
lens of the eye and nerve cells. So if we could block this route, we might be
able to alleviate the damaging effects of glycation.
It is worth looking further into the process of
glycation by fructose, but like all research in this field, this work is
hampered by technical difficulties of how to quantify glycation and
cross-linking. We also need more research into the molecular mechanisms of the
reaction, to clarify the therapeutic potentials of different types of drug.
Meanwhile, for those taking aspirin in low doses to stave off heart attacks,
there may be an unexpected bonus. Perhaps the best advice is to watch the
aspirin story, and keep off the sugary snacks between meals.